The CARC is designed to assess an individual’s risk for adverse outcomes from a COVID-19 infection should one become infected with the SARS-CoV-2 virus (symptomatic or asymptomatic) and the provide the user with suggestions on how to lower that risk, based on best available evidence. COVIDAge is the equivalent age at which a person is expected to have that level of risk for adverse COVID-19 outcomes simply by natural aging.
The risks for COVID-19-related hospitalization, ICU admission, and mortality are determined by the net contributions of multiple modifiable CARC biometrics, most notably those related to hypertension, obesity, diabetes, and coronary heart disease.
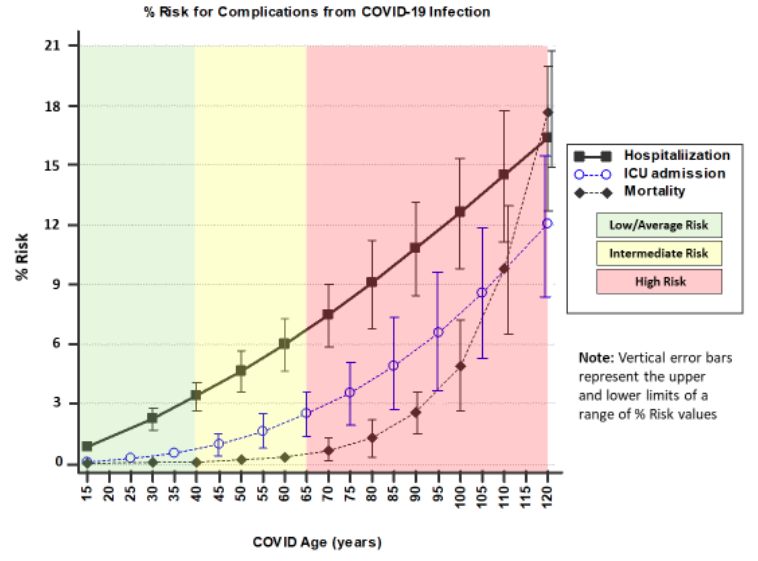
The data underlying the calculated risks for these 3 outcomes (displayed in the ‘Risk Chart’ linked to the CARC; Fig 1) was derived from multiple COVID-19 observational and seroprevalence studies from Mar/Apr 2020 (Bialek et al., Dufort, Chow et al.). Since more recent COVID-19-related data is now available, it is appropriate to confirm that the magnitudes and shapes of the curves in the CARC ‘Risk Chart’ remain valid.
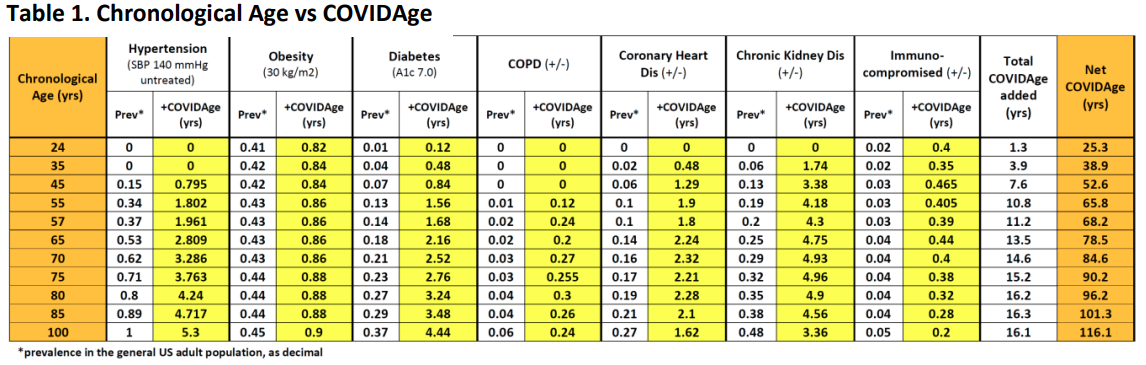
For purposes of this validation, age-specific US prevalence rates for the 7 most significant categories of risk factors in the CARC were defined (Atella et al., Hales et al., Divers et al., American Hospital Association, Centers for Disease Control and Prevention) and matched with the corresponding number of years added by the CARC for someone of that age who has that risk factor (‘added COVIDAge’). The product of the prevalence (as a decimal) and ‘added COVIDAge’ gives an estimate of the ‘average added COVIDAge’ for that risk factor across the US population.
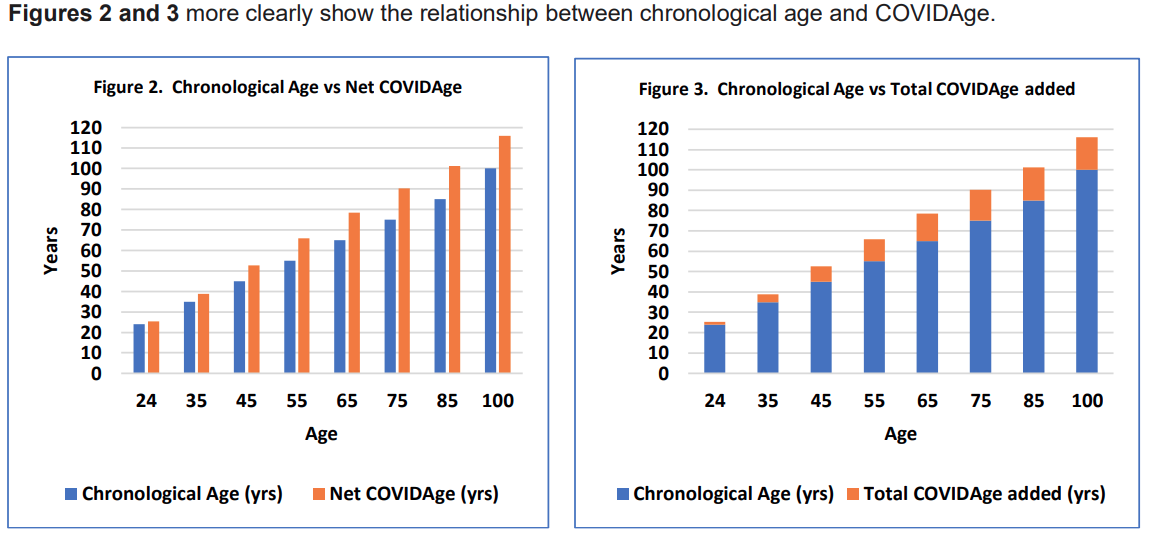
As seen in Table 1, adding together the 7 ‘average added COVIDAges’ (‘+COVIDAges’) for a particular chronological age = ‘Total COVIDAge added.’ Adding the ‘Total COVIDAge added’ to the chronological age = ‘Net COVIDAge,’ i.e., the corresponding COVIDAge for a particular chronological age of an adult who is vulnerable to becoming infected with SARS-CoV-2. For example, the average 55-year-old US adult has a corresponding COVIDAge of 65.8 years, based on the prevalence of risk factors for adverse COVID-19 outcomes in the US population.
Validation of the CARC estimates for the risks of COVID-related hospitalization, ICU admission, and mortality used comparisons of COVIDAges to the most recently publicly available chronological age-specific epidemiological data obtained from US state governmental entities, CDC, and the international literature.
Based on seroprevalence data, it is estimated that, of all New York City residents who were infected with COVID-19 through Jul 2020 (n=1.6 million; 20% of all 8 million residents), 3% (n=55,000) required hospitalization (Levin et al.). According to the CARC, someone aged 37 yrs (median age of all 8 million NYC residents) can be expected to have, on average, a corresponding COVIDAge of 42 yrs, based on the net effect of the age-specific prevalence of the major risk factors for COVID-19 complications. Someone with a COVIDAge of 42 has estimated 3.7 1% risk of hospitalization, closely matching this latest NYC data.
CDC data through Aug 2020 (Centers for Disease Control and Prevention) shows that the rates for hospitalization due to COVID-19 progressively increase by age, relative to those aged 18-29 yrs: 30-39 years: 2x; 40-49 yrs: 3x; 50-64 yrs: 4x; 65-74 yrs: 5x; 75-84 yrs: 8x; 85+ yrs: 13x (Fig 4; blue columns).
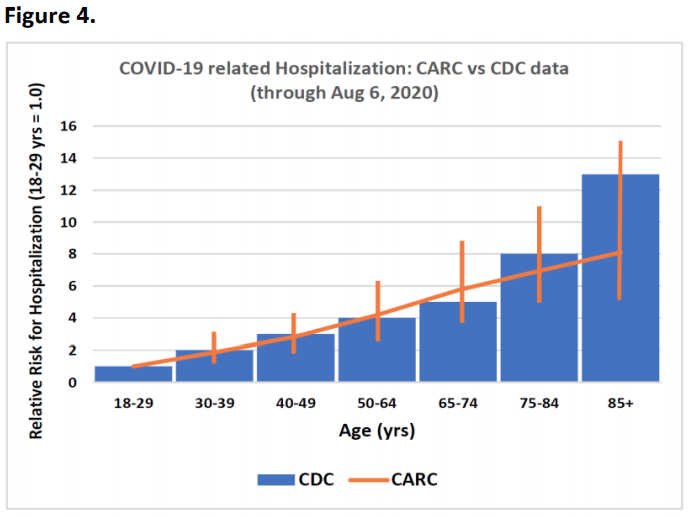
Superimposing the CARC calculated risks for hospitalization on these CDC data columns shows an excellent match (Fig 4; orange trend line with vertical range limits), confirming that the shape of the CARC ‘Hospitalization’ curve in Fig 1 reflects current CDC data.
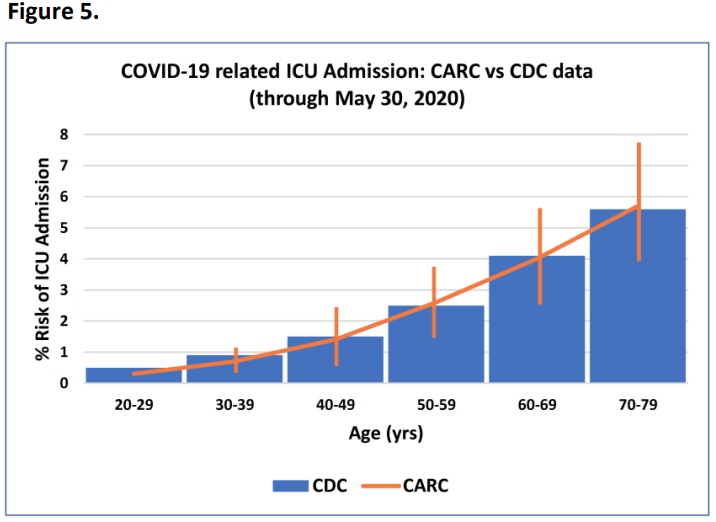
CDC data through the end of May 2020 indicates that, of 1,136,490 confirmed COVID-19 individuals in the US, 25,387 (2.2%) were admitted to an ICU sometime during the course of their disease (Stokes et al.). Fig 5 displays this same data when segregated by age (blue columns).
Superimposing the CARC calculated risks for ICU admission on these CDC data columns in Fig 5 (orange trend line with vertical range limits) shows an excellent match, confirming that both the magnitude and shape of the CARC ‘ICU admission’ risk curve in Fig 1 remain accurate and meaningful.
Because a high proportion of COVID-19 infections are asymptomatic or mildly symptomatic and may not be included in official case reports, it seems most appropriate to compare CARC ‘mortality risks’ to currently available ‘infection fatality rates (IFRs)’, derived from COVID-19 seroprevalence data, rather than to ‘case fatality rates (CFRs)’ (Levin et al.).
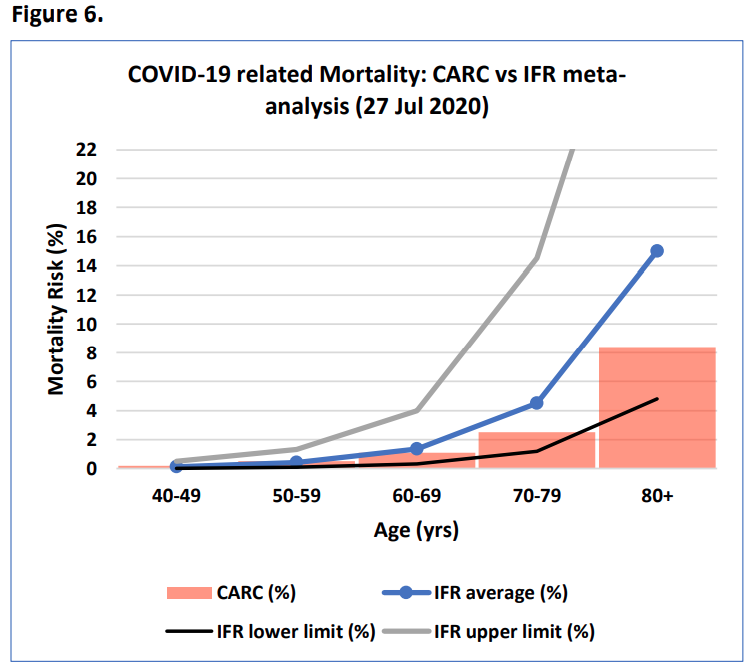
Age-specific meta-regression analysis (Levin et al.) of COVID-19 IFR data from more than 344,000 individuals in 15 countries through Jul 2020, including the USA, is plotted in Fig 6. The average mortality risk for an individual of a certain age who becomes infected with SARS-CoV-2 is linearly plotted together with the upper and lower limits of the reported ‘prediction interval’ for this data.
Comparing these mortality risk numbers of those calculated using the CARC (Fig 6; vertical columns) reveals consistent overlap of values. This indicates that the mortality risks determined using the CARC are reasonable estimates of those recently confirmed at multiple international sites.
Everist Health’s COVIDAge Risk Calculator (CARC) continues to provide valid estimates of the risks for hospitalization, ICU admission, and mortality should an individual become infected with the SARS-CoV-2 virus. This conclusion is based on the most recently available COVID-19-related data from US state governmental entities, CDC, and the international literature.
As the COVID-19 pandemic evolves, so will Everist Health’s CARC, incorporating needed changes to its biometrics and algorithms to provide the user with the most current and accurate assessment of their risks for adverse COVID-19 outcomes.
Although men appear to be at a higher risk for severe complications from COVID-19 infection than women, the reasons for this gender difference remain unclear. Therefore, the COVIDAge Risk Calculator’s % risks for hospitalization, ICU admission, and death are currently based on combined data from men and women.
When more definitive information becomes available that allows separation of the biological from the social behavioral reasons for this difference, the COVIDAge interpretation guidelines will be adjusted accordingly.
Our research.
Our research.
Body Mass Index (BMI) is a rough measure of body fat, by looking at the ratio of weight to height. Despite its limitations for certain groups, BMI is used extensively by governmental agencies including the NIH and CDC in the US. High BMI or Obesity is associated strongly with COVID-19 complications. You can use the Body Weight Scale (Body Weight Scales).
Our research.
Waist circumference is another way to estimate your potential disease risk. Excessive abdominal fat may be serious because it places you at greater risk for developing obesity-related conditions, such as Type 2 Diabetes, high blood pressure, and coronary artery disease.
To correctly measure waist circumference:
Our research.
Smoking strongly contributes to cardiovascular disease, which increases the risk for COVID infection complications.
Our research.
Your Systolic Blood Pressure is the higher number in a blood pressure reading. It is the pressure in your arteries when your heart beats, as opposed to your diastolic blood pressure, which is your arterial pressure between beats. Hypertension, usually defined as persistent Blood Pressure above 130/80 mm/HG, is a strong risk factor for COVID-19 complications. Inexpensive blood pressure monitors available (Blood Pressure Monitors). For more information on low blood pressure: https://bit.ly/3fFcCrg.
Our research.
Everist Health’s COVIDAge Risk Calculator (CARC) is designed to assess an individual’s risk for adverse outcomes from a COVID-19 infection should one become infected with the SARS-CoV-2 virus and to provide the user with suggestions on how to lower that risk, based on best available evidence. It currently does NOT assess an individual’s risk for becoming infected with SARS-CoV-2. The risks for COVID-19 related hospitalization, ICU admission, and mortality are determined based on the cumulative contributions of multiple CARC biometrics, one of which is serum Vitamin D [25(OH)D] level.
Vitamin D (Vit D) is a fat-soluble vitamin that plays a key role in calcium metabolism, neuromuscular and immune function, and reduction of inflammation. Serum Vit D [25(OH)D] insufficiency (20 to <30 ng/ml; 50 to <75 nmol/L) and deficiency (<20 ng/ml; <50 nmol/L) have been strongly linked to poor outcomes from COVID-19 infection, thought to be mainly consequent to associated endothelial dysfunction and the lack of an optimal immune response to the SARS-CoV-2 virus (Ilie et al., Kim et al., Libby et al.).
Among the most notable documented correlations between Vit D insufficiency/deficiency and COVID-19 outcomes are:
Because the COVID-19 pandemic has existed since only the beginning of 2020, the world’s COVID-19 literature is, consequently, predominated by publications from small-scale investigations that report observations and retrospective analyses involving a wide variety of patient populations, both with COVID-19 and/or at risk of contracting it. Although many prospective investigations of the relationship between Vit D and COVID-19 are already, or soon-to-be, underway (48 such studies are current registered in https://ClinicalTrials.gov, half of which are studying the therapeutic benefit of administered Vit D in COVID-19), results from many of these larger studies, unfortunately, may become available only well after the COVID-19 pandemic has become much less of a public health threat – thereby lessening their impact on COVID-19 patient care.
Fortunately, peer-reviewed reports are already beginning to emerge of randomized, prospective Vit D therapeutic studies in COVID-19, along with meta-analyses of recently published investigations of the effect of Vit D on COVID-19 outcomes. Representative of these are:
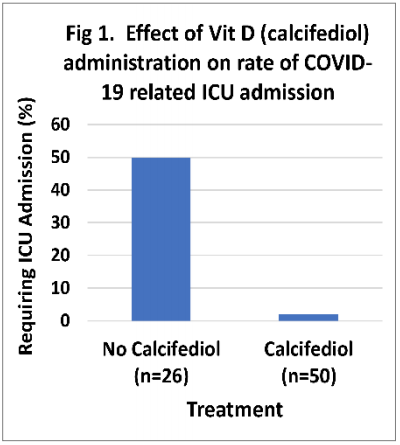
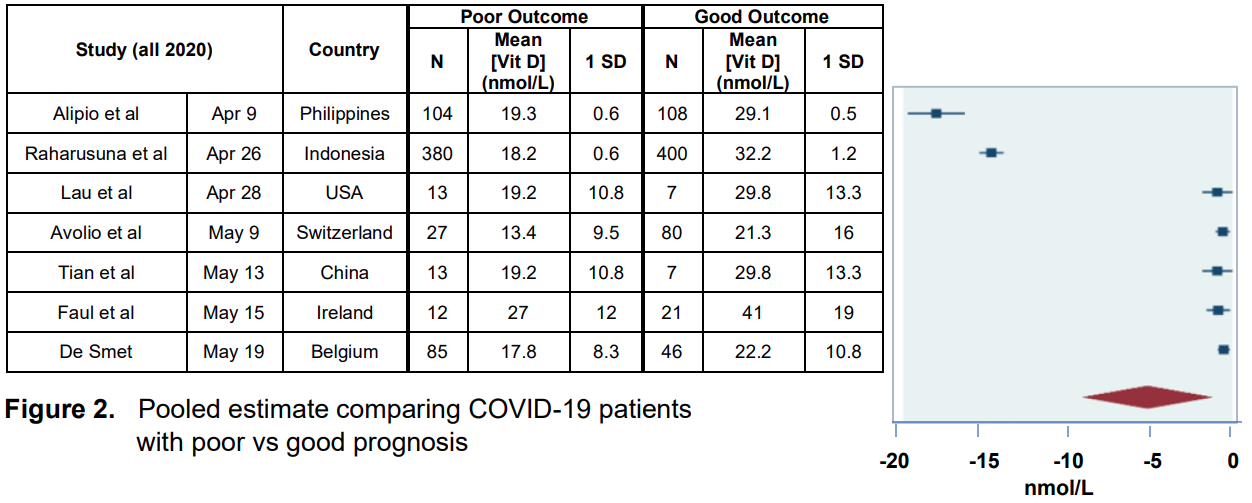
The Vit D biometric in the CARC serves the purpose of providing the user with information about a modifiable health condition that, based on best available scientific and clinical evidence, can be used to help estimate one’s risk for adverse outcomes should that individual become infected with SARS-CoV-2. This is based on strong indirect and, now, emerging direct evidence from multiple international studies, including those highlighted here.
To exclude this biometric from the CARC while awaiting more definitive large-scale studies would deprive the user of receiving information upon which they could quickly act not, at their discretion, while the threat of adverse outcomes to a COVID-19 infection remains most relevant. The potential benefit of avoiding Vit D deficiency clearly outweighs the minimal potential risk of excessive Vit D dosing.
Our research.
The vascular endothelium appears to play a critical role in the pathogenesis of COVID-19. The SARS-CoV-2 virus enters cells through the ACE2 receptor pathway, which is prevalent on endothelial cell surfaces. Early evidence points to endothelial dysfunction as a potential indicator for poor outcomes with COVID-19. AngioDefender is CE Mark approved to measure %FMD of the brachial artery as a non-invasive measure of endothelial function. Click here for more information on the AngioDefender.
*AngioDefender is not FDA approved for this measure.
Questions: info@everisthealth.com.
Our research.
An A1c test determines your average blood glucose level over the last 3 months by measuring the amount of glycolated hemoglobin in your blood. Glycolated hemoglobin is hemoglobin that has been exposed to glucose, so higher percentages mean higher average glucose levels. A healthy A1c is below 5.7, prediabetes is defined by values between 5.7-6.5, and diabetes is indicated by values above 6.5. Diabetes is a major risk factor for COVID-19 complications.
Your can screen yourself at home with tests like the following (At Home HbA1c Tests).
Our research.
High TG (triglycerides) and low HDL-C (high density lipoprotein-cholesterol) blood levels are among the major risk factors for ‘metabolic syndrome,’ a multifactorial disease arising from insulin resistance accompanying abnormal fat deposition and function. Not only does it increase one’s risk for cardiovascular disease but also for complications from COVID-19 infection by promoting systemic inflammation and immune dysfunction. An ideal TG/HDL-C ratio is 2.5 or less when the values for TG and HDL-C are expressed in mg/dL units and less than 1.1 when expressed in mmol/L units.
You can screen yourself at home with tests you can find here or call your provider to schedule a test, as it is usually covered by your insurance plan.
*LDL cholesterol is not calculated into your COVIDAge at this time.
Our research.
Oxidative stress reflects an imbalance between the systemic manifestations of reactive oxygen species (ROS), e.g., free radicals and peroxides, and a biological system’s ability to readily detoxify the resulting reactive intermediates and repair the consequent oxidative damage. By targeting proteins, lipids, and DNA, untethered ROS is thought to be a key factor in the development of a wide variety of diseases and conditions in the body, including cardiovascular diseases (CVD), insulin resistance/diabetes, the unwanted consequences of aging, and, most recently, the pathogenesis and severity of COVID-19 infection.
COVID-19 is caused by the SARS-CoV-2 RNA virus and gains access to humans by binding to the angiotensin-converting enzyme 2 (ACE2), which is abundantly expressed in many human tissues, including lung epithelium, vascular endothelium, heart, kidney, and intestine. What happens next in the pathogenesis of the COVID-19 infection and the emergence of its clinical sequela has been attributed by a variety of investigators to a number of interrelated ‘oxidative stress’ hypotheses.
Cecchini (July 2020) proposes that “oxidative stress plays a role in the pathogenesis of COVID-19, perpetuates the cytokine storm cycle, blood clotting mechanism, and exacerbates hypoxia” (Cecchini et al.). While recognizing that several other viruses induce oxidative stress in order to facilitate their replication inside the cell, these authors propose a pathogenesis model of primary lung injury and late hematological, tissue hypoxemia, and mitochondrial dysfunction due to oxidative stress, based on the body’s analogous responses to other related viral infections.
Derouiche (May 2020) offers a similar explanation for COVID-19 morbidity, linking excessive activation of monocytes/macrophages with the development of a cytokine storm that leads to acute respiratory distress syndrome (ARDS) (Derouiche). This prompted the authors to suggest that increasing the levels of antioxidants, e.g., glutathione (GSH), by dietary polyphenols, e.g., curcumin, is a logical approach to achieving protection against chronic inflammation and oxidant-mediated injury in lung infections like COVID-19.
Prompted by pathological evidence of diffuse endothelial inflammation induced by direct SARS-CoV-2 infection of endothelial cells in the lung, kidney, and small bowel, many investigators are now concluding that SARS-CoV-2 is best characterized as a vasculotropic virus that infects not only the lungs but progressively infects and damages endothelial cells throughout the body (Varga et al., Ackermann et al.). This scenario best explains why some people experience a variety of severe extrapulmonary complications — including blood clots, myocarditis, and neurological symptoms — from a virus that is supposed to just infect the lungs.
This vascular tropism appears to be unique to SARS-CoV-2, since influenza viruses like H1N1 are not known to do this and the original SARS-CoV-1 viral infection was largely confined to the lung. As depicted in Figure 1, after entering the body via ACE2 receptors present on the surface of epithelial cells lining the nose and throat, SARS-CoV-2 moves to the lung alveoli. Subsequent vascular leakage and pulmonary edema are caused by multiple mechanisms:
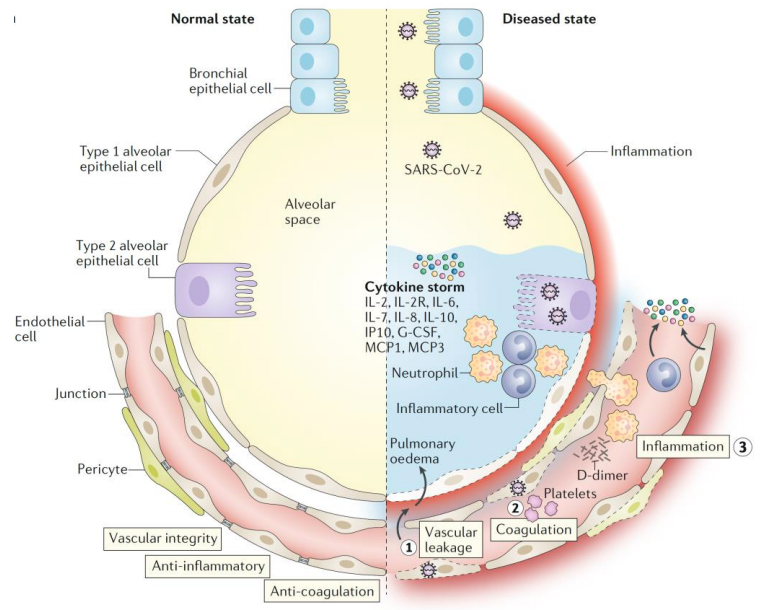
On a background of EDF, endotheliitis likely plays a central role in the development of COVID-19 related venous and arterial thromboembolic phenomena and multi-organ inflammation, including the new pulmonary complication called ‘microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS)’ – an atypical form of ARDS (Mosleh et al., Huertas et al., Ciceri et al.). This progressive syndrome may also involve the microvascular bed of the brain and other vital organs, leading to multiorgan failure and death.
Mounting evidence suggests that pre-existing EDF – associated with obesity, hypertension, diabetes, and other components of metabolic syndrome – could be a sine qua non for developing moderate-to-severe COVID-19 (Ghirga et al.). It follows that pre-existing EDF is likely one of the key underlying conditions that increases the risk for a significant adverse outcome to a COVID-19 infection and that the degree of EDF is positively correlated with the degree of this severity. Measurement of pre-existing EDF, therefore, is likely: 1) vital data for assessing the prognosis of COVID-19 infected patients; 2) a valuable source of information for all people to become more aware of their personal risk for a bad COVID-19 outcome, should they become infected; and 3) a potentially important motivating tool for adoption of a more healthy lifestyle, since most predisposing factors for EDF are modifiable.On a background of EDF, endotheliitis likely plays a central role in the development of COVID-19 related venous and arterial thromboembolic phenomena and multi-organ inflammation, including the new pulmonary complication called ‘microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS)’ – an atypical form of ARDS (Mosleh et al., Huertas et al., Ciceri et al.). This progressive syndrome may also involve the microvascular bed of the brain and other vital organs, leading to multiorgan failure and death.
EDF can be readily and accurately quantified using Everist Health’s patented AngioDefender technology, with the AngioDefender Score put into appropriate perspective regarding how it influences one’s risk for an adverse COVID-19 outcome by employing Everist Health’s COVIDAge Risk Calculator (both accessible at https://everisthealth.com).
Our research.